WRIGHT-PATTERSON AIR FORCE BASE, Ohio – When faced with eminent danger, no one has to think twice about what to do next. Get away from it!
But what if you are in a confined space and maybe not even aware of the danger? That’s a very real possibility faced daily by maintenance technicians every time they go to work inside an aircraft’s fuel tank.
Doing periodic maintenance or repairing components like fuel pumps, wiring or certain wing structures, usually means that someone has to get inside a fuel tank. The current safety procedure is to have another technician outside the tank, staying in constant vocal contact to make sure the worker inside has not succumbed to an exposure to lingering jet fuel vapor—or VOC (volatile organic compound). Unfortunately, by the time everyone is aware of the problem, the damage may already be done, resulting in death or serious injury.
It may seem obvious that using an air monitor might prevent such problems. However, such devices available today are bulky and often hand-held, making them nearly impossible to use while working in confined spaces.
But what if the technician inside the tank had a small, flexible and wearable monitor that would not interfere with physical movements and would emit a signal the moment even a very tiny amount of a toxic gas was detected? Certainly, that technician would stand a good chance of getting out of the fuel tank alive and well.
Air Force research scientists Dr. Ajit Roy and Dr. Nicholas Glavin are developing new strategies for materials that are capable of tackling that problem in AFRL’s Material and Manufacturing Directorate.
“What we are working with is a thin film made of a chemical that is selectively picked to be sensitive to certain gas molecules,” said Roy. “When a molecule of the gas comes close to the surface of the film, it changes the electrical property of the film’s material. That change is what gets detected.”
“When that happens, the detector sends an RF (radio frequency) signal to a wifi receiver, which alerts the technician,” said Roy.
The idea is to make the detector extremely lightweight and flexible so that it might even be sewn into the fabric of a technician’s clothing. Such a detector would not interfere with physical movements or—should the need arise—a quick exit from the confined space.
The detector chemical currently under investigation is molybdenum disulfide—the mineral molybdenite, which forms naturally in thin sheets. Each sheet comprises a single layer of molybdenum atoms sandwiched and chemically bound between a layer of sulfur atoms. These sheets can slide easily over one another, making molybdenite an ideal lubricant—its most common industrial use. This same molecular arrangement, however, makes it nearly perfect for use as a toxic agent detector.
A device like this also has applications beyond protecting aircraft technicians working in fuel tanks. Warfighters in the field who may be exposed to weaponized airborne toxic agents, for example, could use this technology. The catch is that the detector’s material, molybdenum disulfide, has to be “doped” with another chemical to make it sensitive to a different gas. As a result, it can detect only what it is designed to detect.
That’s the bad news. The good news is that many such detectors can be put on the same small, flexible film. Instead of carrying a massive or bulky detection apparatus into the field, a warfighter might be wearing it like a fitbit or as a patch sewn onto a sleeve. When asked about the monetary cost of outfitting each individual warfighter with a detector, Roy described the device as being “very affordable.”
Roy expects the same principles to eventually be applied to biological and well as radiological agents. Although such detectors currently exist, not only are they large, but they also do not provide a “real-time” alert. For example, a dosimeter is typically worn for as long as a month and then checked to see if the wearer has been exposed to radiation. By the time the exposure has been detected, it might have been an on-going event occurring over several days or weeks—and possibly still occurring. That delay and prolonged exposure would be avoided with a real-time device like the one Roy and his colleagues are working on.
For now, however, Roy and Glavin are concentrating on making the VOC detector work. Although a thin-film, flexible device is still under development, a larger device using the same chemical technology has been successfully tested in a laboratory gas chamber. It is hoped that the smaller flexible version will be available within a year.
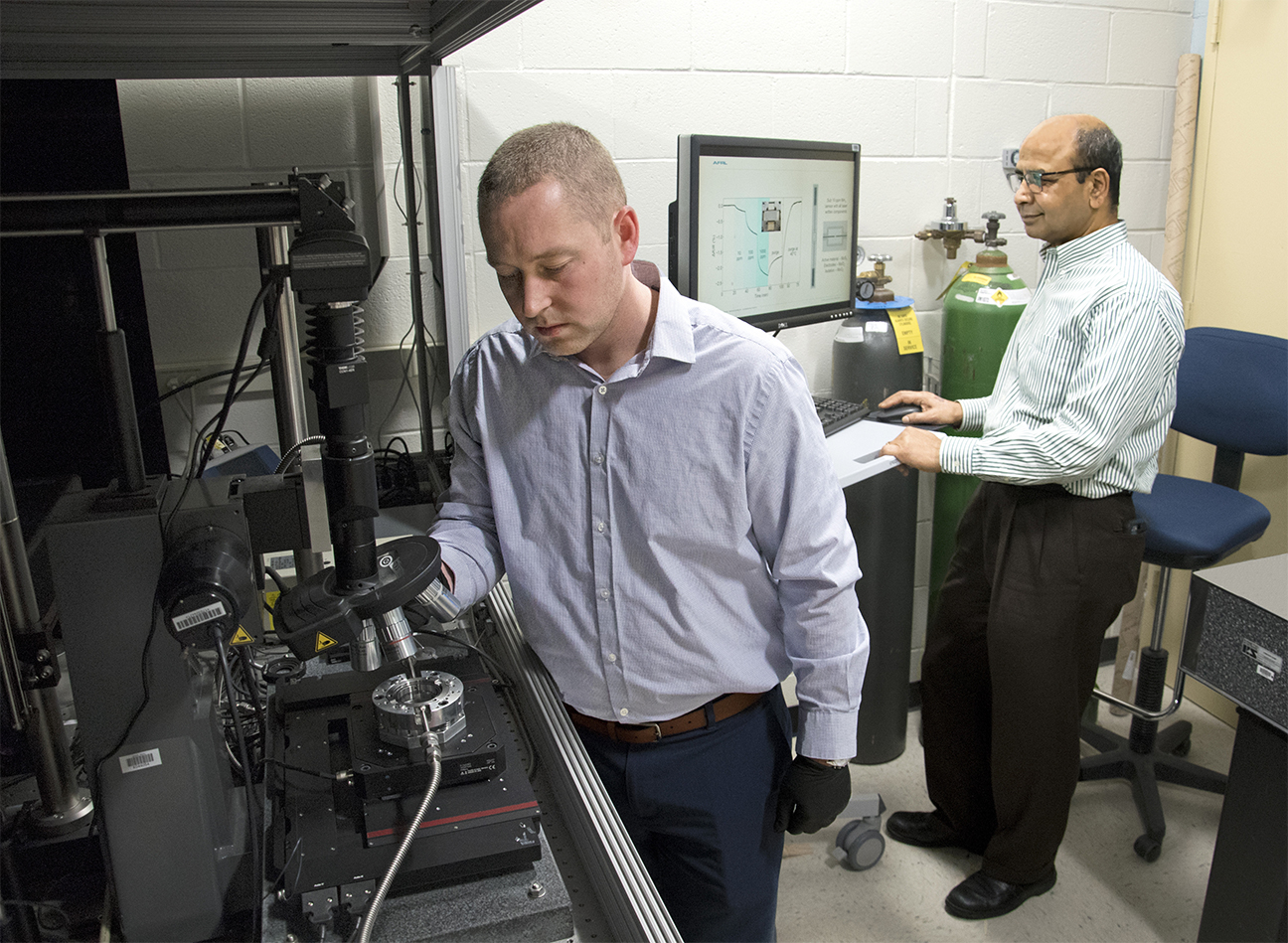
Researchers Dr. Nicholas Glavin (left) and Dr. Ajit Roy are working to develop flexible, wearable toxic agent detectors. (U.S. Air Force photo/Spencer Deer)

Current state-of-the-art for aircraft confined space maintenance (Courtesy photo)